The following pictures represent solutions at various stages in the titration of a weak diprotic acid H2A with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A2- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity) . 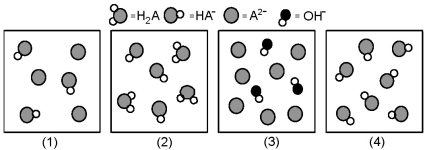
-Which picture represents the system with the highest pH?
Definitions:
GDP
Stands for Gross Domestic Product, a measure of the economic performance of a country, representing the total value of all goods and services produced over a specific time period.
Phosphorylated
Describes a molecule that has undergone phosphorylation, the addition of a phosphate group, often modifying its function or activity.
Glycolysis
The first step in cellular respiration, a pathway that converts glucose into pyruvate, generating ATP and NADH.
Citric Acid Cycle
A series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats, and proteins.
Q8: At 298 K,K<sub>c</sub> = 1.7 × 10<sup>-56</sup>
Q25: Which pair of ions can be separated
Q62: The standard potential for the following galvanic
Q91: The percent dissociation of acetic acid changes
Q95: What is the Henderson-Hasselbalch equation for the
Q130: Standard molar entropies,S°,in J/K∙mol,are given below each
Q135: For the hypothetical reaction A + B<sup>x</sup>
Q138: Sulfurous acid,H<sub>2</sub>SO<sub>3</sub> has acid dissociation constants K<sub>a1</sub>
Q166: What is the relationship between the standard
Q197: Consider the following standard reduction potentials, Zn<sup>2+</sup>(aq)+