The following pictures represent solutions at various stages in the titration of a weak diprotic acid H2A with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A2- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity) . 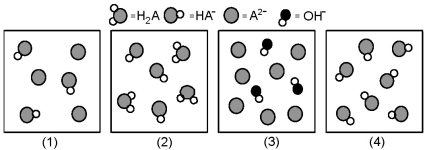
-Which picture represents the system with the lowest pH?
Definitions:
Marginal Product
The additional output that results from using one more unit of a particular input, while holding other inputs constant.
Average Product
The output obtained by dividing the total product by the number of units of the variable input used in production, ideally reflecting productivity per input unit.
Total Product
The total output or quantity of goods produced by a firm or economy at a given level of input.
Diminishing Returns
A principle stating that if one factor of production is increased while other factors are held constant, the incremental increases in output will eventually decrease.
Q18: Which picture represents a neutral salt?<br>A)(1)<br>B)(2)<br>C)(3)<br>D)None of
Q30: Calculate K<sub>sp</sub> for PbI<sub>2</sub> at 25°C based
Q42: How many grams of nickel metal are
Q59: Of the elements indicated on the periodic
Q64: Which one of the following salts,when dissolved
Q66: If ΔG° is positive for a reaction,<br>A)K
Q69: Oxalic acid,H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> has acid dissociation constants K<sub>a1</sub>
Q96: The neutralization constant K<sub>n</sub> for the neutralization
Q103: K<sub>c</sub> = 57.0<sup> </sup>at 700 K for
Q156: Calculate the pH of a 0.060 M