The figure below represents the spontaneous reaction of H2 (shaded spheres) with O2 (unshaded spheres) to produce gaseous H2O. 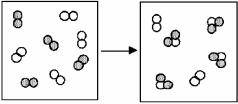
-How will the spontaneity of this reaction vary with temperature? This reaction is
Definitions:
Material Cost
The cost associated with the raw materials and components required for the manufacturing of a product.
Ordering Cost
Expenses associated with placing orders for additional inventory, including administrative costs and the costs of processing and receiving orders.
Holding Cost
The expenses associated with storing unsold goods or materials, including warehousing, insurance, and depreciation costs.
Order Cost
The total expense incurred in making and processing an order, including any procurement, delivery, and handling costs.
Q6: For any thermodynamic function Y,ΔY° for a
Q23: What is the pH of a 0.100
Q48: What is the identity of M in
Q50: In the reaction between NO<sub>2</sub> and H<sub>2</sub>O
Q81: What is the pH of a solution
Q94: What is the pH of the resulting
Q131: What is the pH of a solution
Q154: Using the conjugate acid-base pairs listed below,complete
Q179: Determine the number of water molecules necessary
Q193: Which point a-d represents the second equivalence