Calculate the standard free energy for the reaction given. 2 CH3OH(l) + 3 O2(g) → 2 CO2(g) + 4 H2O(l) 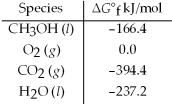
Definitions:
Our Common Future
A report published by the World Commission on Environment and Development (WCED) in 1987, also known as the Brundtland Report, focusing on sustainable development.
Anthropocentrism
The belief that human beings are the most important entity in the universe, often leading to viewing non-human life forms and nature from a human-centered perspective.
Ecological Modernization
A theory proposing that economies can continue to grow while simultaneously reducing their environmental impact, through technological innovation and improved environmental governance.
Ecofeminism
An approach that investigates the domination of women and nature by men.
Q42: How many grams of nickel metal are
Q58: What is the shorthand notation that represents
Q64: What is the pH of a solution
Q69: Oxalic acid,H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> has acid dissociation constants K<sub>a1</sub>
Q71: Ag<sup>+</sup>(aq)+ e<sup>-</sup> → Ag(s)E° = +0.800 V
Q95: If the age of the Earth is
Q120: Calculate the solubility (in g/L)of silver carbonate
Q131: Sodium hypochlorite,NaOCl,is the active ingredient in household
Q166: What is the pH at the first
Q187: Which of these neutralization reactions has a