The figure below represents the spontaneous reaction of H2 (shaded spheres) with O2 (unshaded spheres) to produce gaseous H2O. 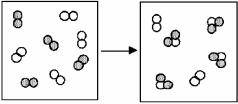
-How will the spontaneity of this reaction vary with temperature? This reaction is
Definitions:
EPS Growth Rate
The rate at which a company's earnings per share (EPS) has grown or is expected to grow over a specified period.
Rate of Return
The positive or negative financial outcome of an investment over a specific period, expressed in terms of a percentage of the cost of investment.
EPS
EPS stands for Earnings Per Share, a financial ratio that indicates the portion of a company's profit allocated to each outstanding share of common stock.
Marginal Tax Bracket
The tax rate that applies to the last dollar of the taxpayer's income, indicating additional taxes on future earnings.
Q32: What are the signs (+,or -)of ΔH,ΔS,and
Q37: Which reaction below represents <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt="Which
Q76: Which of the following processes is spontaneous?<br>A)a
Q82: What is the molarity of a potassium
Q116: The chemical system shown below is at
Q130: Which of the following buffer solutions will
Q145: When the equation below is balanced in
Q153: The pH of 0.150 M CH<sub>3</sub>CO<sub>2</sub>H,acetic acid,is
Q163: For a galvanic cell that uses the
Q183: Which cell involves a nonspontaneous redox reaction?<br>A)concentration