Consider the following gas-phase reaction of A2 (shaded spheres) and B2 (unshaded spheres) :
A2(g) + B2(g) ⇌ 2 AB(g) ΔG ° = +25 kJ 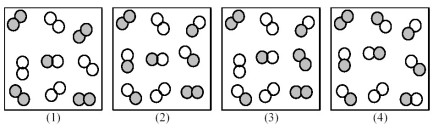
-Which of the above reaction mixtures has the least spontaneous forward reaction?
Definitions:
Cartilage
A flexible, rubbery connective tissue found in various parts of the body, such as the joints, ear, and nose, providing support and structure.
Macrophage
A type of white blood cell that engulfs and digests cellular debris, foreign substances, microbes, and cancer cells.
Phagocytizes
The process by which cells engulf and digest solid particles, typically used by immune system cells to eliminate pathogens and debris.
Infections
The entry and proliferation of microorganisms like bacteria, viruses, and parasites, which are usually absent from the body, frequently leading to sickness.
Q8: Which of the following statements about gamma
Q24: At constant pressure and temperature,which statement is
Q59: Using the following reduction potentials for copper
Q74: For the hypothetical reaction 3 A +
Q104: What volume of 5.00 × 10<sup>-3</sup> M
Q104: Radiation is dangerous to organisms because<br>A)all radionuclides
Q106: What is the selenide ion concentration [Se<sup>2-</sup>]
Q122: What is the ground-state electron configuration for
Q134: What is the approximate value of the
Q205: Identify the Br∅nsted-Lowry bases.<br>A)(1)and (3)<br>B)(1)and (4)<br>C)(2)and (3)<br>D)(2)and