Consider the following gas-phase reaction of A2 (shaded spheres) and B2 (unshaded spheres) :
A2(g) + B2(g) ⇌ 2 AB(g) ΔG ° = +25 kJ 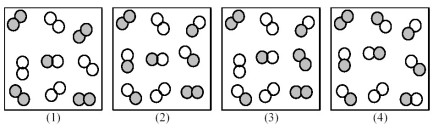
-Which of the above reaction mixtures has the most spontaneous forward reaction?
Definitions:
Dividends Payable
A liability representing an amount owed by a company to its shareholders as a distribution of the company's earnings.
End of the Year
Typically refers to the close of the fiscal or calendar year, a key time for financial reporting and assessment for businesses.
Depreciation Expense
The allocation of the cost of a tangible asset over its service life, representing how much of an asset's value has been used up over time.
Accounts Receivable
Unpaid amounts from customers for goods or services that a business has already provided.
Q2: For the hypothetical reaction A + B<sup>x</sup>
Q7: Which of the following should have the
Q31: How many grams of nickel metal are
Q41: In which of the following solutions would
Q61: Calculate ΔS° for the following reaction. N<sub>2</sub>(g)+
Q68: What is the molar solubility of Mg(OH)<sub>2</sub>
Q70: Which point a-d represents the HX<sup>-</sup>/X2<sup>-</sup> buffer
Q99: The equilibrium constant,K,can be calculated from<br>A)E°.<br>B)E.<br>C)either E°
Q108: Fluorine-18 is an isotope used in Positron
Q181: What is the pH of a solution