Consider the following gas-phase reaction of A2 (shaded spheres) and B2 (unshaded spheres) :
A2(g) + B2(g) ⇌ 2 AB(g) ΔG ° = +25 kJ 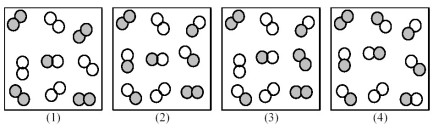
-Which of the above reaction mixtures is ΔG of reaction = ΔG °?
Definitions:
Processing Costs
Expenses associated with the process of transforming raw materials into finished goods, often used in industries where products undergo many stages of production.
Sales and Direct Cost
The total revenue generated from goods and services minus the direct costs associated with producing those goods and services.
Activity-Based Costing
A method of costing that identifies the relationship between costs, activities, and products, and through this relationship, assigns indirect costs to products less arbitrarily than traditional methods.
Processing Costs
The expenses involved in handling, treating, or converting raw materials into finished products.
Q12: Which is a net ionic equation for
Q45: What is the strongest acid among the
Q65: What is the shorthand notation that represents
Q66: What is the characteristic pH-titrant curve for
Q70: In the shorthand notation for a galvanic
Q75: Which is the strongest oxidizing agent under
Q123: Calculate the pH of a 0.20 M
Q135: The total binding energies for <sup>3</sup>He,<sup>4</sup>He,and <sup>6</sup>He
Q149: The nuclear transformation potassium-40 argon-40 + ?
Q175: Based on the half-reactions and their respective