The initial concentrations of Ag+(aq) and Cu2+(aq) are both 1.0 M.What will happen to the cell voltage if 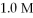 Cu(NO3) 2 is added to the compartment containing the 1.0 M Cu2+(aq) ? The cell voltage will
Cu(NO3) 2 is added to the compartment containing the 1.0 M Cu2+(aq) ? The cell voltage will
Definitions:
Income Statement
A financial document that shows a company's revenues and expenses over a specified period, illustrating how the net revenue of the company is transformed into net income.
Dividend Yield Ratio
A financial ratio that measures the amount of dividends an investor is expected to receive for each dollar invested in a company's stock, expressed as a percentage.
Market Price
The current price at which an asset or service can be bought or sold in the open market.
Dividends on Common Stock
Payments made to shareholders out of a company's earnings, specifically to the holders of its common stock.
Q54: The mass defect for the chemical reaction
Q65: What is the expected order for increasing
Q94: Which of the following steps is not
Q109: Based on the variation in Z<sub>eff</sub>,which oxoanion
Q113: In a nuclear reaction,the symbol for a
Q121: The oxidation state of palladium in K<sub>2</sub>[PdCl<sub>4</sub>]
Q125: Which of the following isotopes is the
Q131: When zinc sulfide undergoes roasting,the products are<br>A)Zn
Q141: What kind of decay process is occurring
Q169: Given that E°<sub>red</sub> = -0.26 V for