Shown below is an electrochemical cell with anode a and cathode c.Both the anode and the cathode are inert electrodes.The liquid shown in compartment b of the cell is molten potassium chloride,KCl(l) . 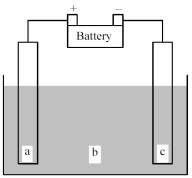
-Determine whether this is a galvanic or an electrolytic cell and give the reaction occurring at the anode and the reaction occurring at the cathode.
Definitions:
Insurance Premiums
Payments made to an insurance company to buy and maintain an insurance policy, typically paid monthly, quarterly, or annually.
Deductible
A deductible is an amount paid by an insured party before the insurance company covers the remaining costs of a claim.
Dependent
A person, often a child or elderly family member, whose livelihood and financial support are largely provided by someone else, typically a family member.
Doctor Bills
Invoices or financial charges for medical services rendered by a physician.
Q16: Describe what happens when 3.0 M NH<sub>3</sub>
Q26: Chemical and physical changes can be classified
Q32: What are the signs (+,or -)of ΔH,ΔS,and
Q36: Nuclei that are in the band of
Q40: A small block of wood or heavy
Q50: How many d electrons are there in
Q57: Formic acid (HCO<sub>2</sub>H,K<sub>a</sub> = 1.8 × 10<sup>-4</sup>)is
Q178: In a galvanic cell,the half-reaction MnO<sub>4</sub><sup>-</sup>(aq)+ 8
Q183: Which cell involves a nonspontaneous redox reaction?<br>A)concentration
Q212: The galvanic cell represented by the shorthand