Based on the balanced chemical equation shown below,determine the mass percent of Fe3+ in a 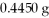 sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is: 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is: 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
Definitions:
Q15: The half equivalence point in the titration
Q18: Which of the following nuclides is most
Q19: What is the equation relating the equilibrium
Q23: "Isotopes" are atoms with the same number
Q37: The nickel-cadmium battery cell has a standard
Q73: What is the shorthand notation for the
Q100: What are the coefficients in front of
Q156: In the unbalanced equation shown below how
Q157: What are the coefficients in front of
Q171: A few sheets of ordinary paper can