Consider the following galvanic cell. 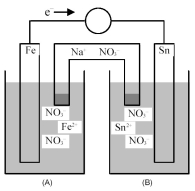
-Identify the anode and cathode,and indicate the direction of ion flow to and from each electrode.
Definitions:
Depressant
A substance that reduces the functioning of the nervous system, often used to decrease anxiety and induce sleep.
Narcotic
A class of substances that relieve pain and induce a state of sleep or stupor; often associated with opioids, which can be both legal medications and illicit drugs.
Barbiturates
A class of drugs that are used as sedatives and hypnotics, which can depress the central nervous system.
Drug Interaction
A modification in the effect of a drug when it is taken in combination with another drug, leading to increased efficacy, reduced effectiveness, or adverse side effects.
Q14: Which of the following are unstable with
Q33: Which of the following is true?<br>A)As a
Q45: For which of these solutions is pH
Q51: For initial state 1 what is the
Q67: Consider a buffered solution consisting of H<sub>2</sub>CO<sub>3</sub>
Q105: Which of the following processes will decrease
Q120: Calculate the solubility (in g/L)of silver carbonate
Q150: Is the cell shown above a galvanic
Q170: The pH of a 0.150 M formic
Q197: Consider the following standard reduction potentials, Zn<sup>2+</sup>(aq)+