The initial concentrations of Ag+(aq) and Cu2+(aq) are both 1.0 M.What will happen to the cell voltage if 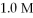 Cu(NO3) 2 is added to the compartment containing the 1.0 M Cu2+(aq) ? The cell voltage will
Cu(NO3) 2 is added to the compartment containing the 1.0 M Cu2+(aq) ? The cell voltage will
Definitions:
Shared Vision
A common understanding and agreement on future goals and direction among members of a group or organization.
Cultural Esteem
The respect, value, and honor given to specific cultures or cultural practices by individuals or societies.
Assertiveness
A measure of how competitive and aggressive a culture is.
Altruism
The principle or practice of concern for the welfare of others, often at a cost to oneself.
Q5: Which has the highest melting point?<br>A)La<br>B)W<br>C)Os<br>D)Hg
Q18: Which of the following nuclides is most
Q63: What percentage of a radioactive substance remains
Q67: ΔS° = -198.7 J/K for the reaction
Q82: The number of neutrons in <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"
Q94: Which process decreases the neutron/proton ratio?<br>A)alpha emission<br>B)beta
Q113: What is the balanced equation for the
Q142: Reduction half-reactions with corresponding standard half-cell potentials
Q144: The balanced net ionic equation for the
Q150: Which is the lowest at 25°C?<br>A)ΔG°<sub>f</sub> for