Consider the following galvanic cell. 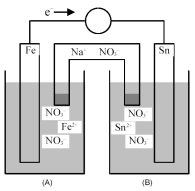
-What is the quantitative change in the cell voltage on increasing the ion concentration in the anode compartment by a factor of 10?
Definitions:
Columbine High School
The site of a tragic mass shooting that occurred on April 20, 1999, in Littleton, Colorado, bringing attention to issues of gun control and school safety.
Littleton, Colorado
A city located in the state of Colorado, United States, known for its suburban setting and as the site of Columbine High School, where a notable school shooting occurred in 1999.
School Violence
Any form of violent behavior occurring within educational institutions.
Teenage Violence
Refers to aggressive and violent behaviors exhibited by individuals in their teenage years, which can include physical fights, bullying, and other forms of aggression.
Q23: How many unpaired electrons are present in
Q36: Shown below are the reactions occurring in
Q48: Consider the galvanic cell, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt="Consider
Q80: A positive value of ΔG°<sub>f</sub> for a
Q87: Standard free energies of formation,ΔG°,in kJ/mol,are given
Q91: <sup>201</sup>Tl is used in myocardial perfusion imaging.It
Q92: Energy is _ (released,required)in the transmutation reaction
Q106: A galvanic cell consists of a La<sup>3+</sup>/La
Q184: What species is oxidized in the reaction:
Q193: The equilibrium constant for the reaction below