Shown below is an electrochemical cell with anode a and cathode c.Both the anode and the cathode are inert electrodes.The liquid shown in compartment b of the cell is molten potassium chloride,KCl(l) . 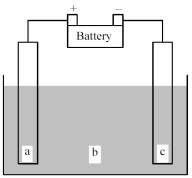
-Determine whether this is a galvanic or an electrolytic cell and give the reaction occurring at the anode and the reaction occurring at the cathode.
Definitions:
Discontinued Operations
Parts of a company's operations that have been sold or terminated, which are reported separately from continuing operations on financial statements.
Tax Rate
The percentage at which an individual or corporation is taxed.
Partial Income Statement
A financial document that reports a company's revenues, expenses, and profits over a portion of the fiscal year, rather than the entire year.
Working Capital
The measure of a company's operational liquidity, calculated as current assets minus current liabilities.
Q4: Entropy is a measure of<br>A)free energy.<br>B)the heat
Q5: Galvanized steel is steel coated with a
Q28: According to the balanced equation shown below,1.00
Q39: What are the coefficients in front of
Q69: Oxalic acid,H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> has acid dissociation constants K<sub>a1</sub>
Q91: Based on the half-reactions and their respective
Q102: Consider the reaction: N<sub>2</sub>(g)+ 3 F<sub>2</sub>(g)→ 2
Q108: Which point a-d represents the first equivalence
Q135: If solution (1)is a saturated solution of
Q216: One way to prepare a solution with