Based on the balanced chemical equation shown below,determine the mass percent of Fe3+ in a 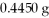 sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is: 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is: 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
Definitions:
Reflexes
Involuntary and nearly instantaneous movements in response to stimuli, highlighting the nervous system's role in bodily function.
Childhood Experiences
Events or circumstances encountered during one's early years of life that can significantly influence behavior and development.
Empirically
In a manner based on observation or experience rather than theory or pure logic.
Personality Traits
Inherent characteristics that influence an individual's patterns of thoughts, feelings, and behaviors.
Q24: Which one of the following objects is
Q86: Based on the following information, Cl<sub>2</sub>(g)+ 2
Q99: Which Pt(II)complexes are geometric isomers of each
Q109: A buffer solution is prepared by dissolving
Q109: Which has the highest standard molar entropy
Q137: Barium hydroxide is slightly soluble in water,with
Q146: A steel pipe can be protected from
Q151: Which of the following can function as
Q178: Which has the lowest melting point?<br>A)Sc<br>B)V<br>C)Mn<br>D)Zn
Q211: Determine the number of water molecules necessary