The mass change for the reaction shown below is ________ amu.The atomic masses are 3He (3.0160),4He (4.0026),1H (1.0078).
2 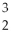 He →
He → 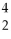 He + 2
He + 2 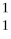 H
H
Definitions:
Units Transferred Out
The number of units moved from one production process or department to the next stage or to finished goods inventory.
Weighted-Average Cost Method
A method of costing inventory that determines the cost of goods sold and the value of the ending inventory by averaging the cost of all available units over the period.
Equivalent Units
A concept in cost accounting used to allocate costs to partially completed goods, converting them into the amount of finished goods units that could have been produced.
Conversion Costs
The combined costs of direct labor and overhead associated with transforming materials into finished goods.
Q10: For a galvanic cell,the cathode has a
Q20: Identify the electrodes and the direction of
Q35: Which of the above are bidentate ligands?<br>A)All
Q58: The net ionic reaction of solid CaH<sub>2</sub>
Q69: Write a balanced chemical equation for obtaining
Q115: Which of these elements is likely to
Q115: Which is not generally considered to be
Q125: Using shorthand notation,the electron configuration of Co<sup>3+</sup>
Q129: How is slag separated from molten ore
Q137: The balanced chemical equation for the production