When butane, 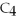
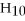 ,reacts with oxygen to produce carbon dioxide and water,2880 kJ of heat are released per mole of methane.How many kJ of heat are released per gram of oxygen used?
,reacts with oxygen to produce carbon dioxide and water,2880 kJ of heat are released per mole of methane.How many kJ of heat are released per gram of oxygen used?
Definitions:
Master Production Schedule
A detailed plan that outlines the specific quantities and timing of finished products to be produced, directly influencing procurement, manufacturing operations, and inventory levels.
Bill Of Materials
A comprehensive list detailing the raw materials, components, and assemblies required to create a product.
Materials Required
The list of all items and substances needed to complete a manufacturing process, project, or operation.
Planning Bills
A material grouping created in order to assign an artificial parent to a bill of material; also called “pseudo” bills, kitted material, or kits.
Q6: Which one of the following elements forms
Q23: The property that describes the ease with
Q34: Which set of properties would identify an
Q69: What is the empirical and molecular formulas
Q73: Which characteristics correctly describe a proton?<br>A)approximate mass
Q95: In the protein,Val-Tyr-His-Pro,which amino acid contains the
Q97: How many isomers are there for C<sub>5</sub>H<sub>12</sub>?<br>A)3<br>B)4<br>C)5<br>D)6
Q163: To which family does this organic compound
Q167: Using the above models of hydrides,indicate the
Q233: Identify the amino acid shown below. <img