Diatomic nitrogen is added to the equilibrium system:
N2 (g) + H2 (g) 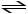
2 NH3(g) + heat
When a new equilibrium is established the concentration of H2 will be ________ the amount at the original equilibrium,and the amount of NH3 will be ________ the amount at the original equilibrium.
Definitions:
Social Consequence
The results or implications that actions, behaviors, or policies have on society at multiple levels, ranging from individuals to broader communities.
Criminal Offenses
Acts or actions that are considered illegal by law and are punishable by the judicial system.
Chinese Drinking Culture
The customs, practices, and social functions of consuming alcoholic beverages within Chinese society.
Drunkenness
A state of intoxication caused by the consumption of excess alcohol, impairing cognitive and physical functions.
Q2: Which reaction illustrates artificial transmutation by alpha
Q5: Calculate the hydrogen ion concentration in a
Q15: Which molecule can have cis-trans isomers?<br>A)CH<sub>3</sub>CH=C(CH<sub>3</sub>)<sub>2</sub><br>B)(CH<sub>3</sub>)<sub>2</sub>C=CHCH<sub>3</sub><br>C)(CH<sub>3</sub>)<sub>2</sub>C=C(CH<sub>3</sub>)<sub>2</sub><br>D)CH<sub>3</sub>CH=CHCl<br>E)CH<sub>3</sub>CH=CCl<sub>2</sub>
Q20: Which measurement represents the smallest quantity?<br>A)2950 ng<br>B)2.95
Q24: Caffeine,the active ingredient in coffee,is composed of
Q39: The electron configuration for phosphorus is<br>A)1s<sup>2 </sup>1p<sup>6
Q45: How many carbon atoms are there in
Q47: The HCO<sub>3</sub> -1 ion is called _.<br>A)hydrogen
Q67: The formula PO<sub>4 </sub><sup>3-</sup> means that this
Q95: What is the normality of a solution