Consider the reaction shown and identify the statement that is not true.
CaCO3(s) 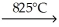 CaO(s) + CO2(g)
CaO(s) + CO2(g)
Definitions:
Current Liability
A company's debts or obligations that are due to be paid within one year or within the normal operating cycle.
Pension Expense
The cost recognized by a company for the benefits provided to employees in retirement pension plans.
Fully Funded Pension Rights
Retirement plan benefits that have been completely backed by financial contributions, ensuring that sufficient assets are available to meet future obligations.
Pension Expense
The total amount an employer is obligated to contribute to an employee's defined benefit pension plan in a given period.
Q9: LX(aq)+ MY(aq)→ LY(s)+ MX(aq)<br>A)redox<br>B)acid-base<br>C)precipitation
Q22: The molar mass of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4943/.jpg" alt="The
Q34: All of the following can be found
Q50: If "A" contains 2% NaCl and is
Q51: When the reaction shown is correctly balanced,the
Q54: A molecule in which the central atom
Q55: A chemical bond formed between two identical
Q59: Explain why it is unlikely that an
Q83: Which solution will have the highest boiling
Q87: Which assumption of Kinetic Molecular Theory is