2 SO2(g) + O2(g)  2 SO3(g) + heat K = 4.8 ×
2 SO3(g) + heat K = 4.8 × 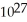
Which statement about this system is not true?
Definitions:
Exchange
The act of trading goods, services, or ideas between two parties.
Core Tactic
The primary or most essential strategy used to achieve an objective.
Consultation
The process of seeking expertise and advice from others to inform decision-making or to enhance understanding.
Inspirational Appeal
A persuasive tactic that aims to motivate or inspire others by appealing to their emotions and values.
Q3: A solution containing 145.0 g AgNO<sub>3</sub> is
Q10: The number of valence electrons in the
Q17: The process of sublimation is _ and
Q22: oxygen difluoride<br>A)ICl<br>B)C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4943/.jpg" alt="oxygen difluoride A)ICl
Q28: The molar mass of Fe(OH)<sub>3</sub> is _
Q46: Which chemical symbol represents a non-metal?<br>A)Al<br>B)B<br>C)Ga<br>D)Si<br>E)P
Q57: Which statement is correct for pure water?<br>A)Pure
Q61: Which group of elements contains only non-metals?<br>A)Mg,Ca,Sr<br>B)V,Cr,Mn<br>C)Cl,Ar,K<br>D)P,As,Se<br>E)C,S,I
Q66: What is the final concentration of a
Q79: The functional group illustrated by R-OH is