In a reversible heat engine, one mole of an ideal gas is carried through a circular cycle beginning and ending at point A as shown in the figure. Which one of the following statements concerning this system is false? 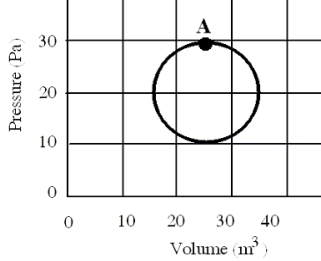
Definitions:
Price
The sum required, expected, or disbursed as a purchase price for an item.
Government-Provided Goods
Goods and services offered to the public by the government that might not be provided by the market or would be underprovided, such as public parks and national defense.
Explicit Fee
A clear and defined charge for a service or product that is not hidden or implicit.
Antipoverty Programs
Government or organizational initiatives designed to reduce or eliminate poverty by providing assistance to the needy through services or financial aid.
Q3: Which one of the following statements is
Q3: Which one of the following combinations of
Q15: At what rate is heat lost through
Q24: Water is flowing through a rectangular channel
Q35: What is the internal energy of 1.75
Q38: A 2.0-g sample of steam at 100
Q41: A paddle wheel frictionally adds thermal energy
Q61: What is the final potential difference across
Q62: Elaine went to her outside faucet to
Q99: Determine the length of a copper