Beneath the moveable piston in the drawing,2.25 moles of a monatomic ideal gas is enclosed at 314 K. The initial volume of the gas is 3.0 m3. The gas is compressed isothermally to a final volume of 1.0 m3. How much heat is removed from the gas during this process? 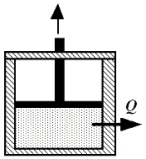
Definitions:
IFRS 3
IFRS 3 is an International Financial Reporting Standard that provides guidance on accounting for business combinations, requiring entities to measure the acquiree's assets and liabilities at their fair values at the acquisition date.
Liabilities
Financial obligations a company owes to external parties, including loans, accounts payable, and other debts.
Business Combinations
Mergers and acquisitions where one company acquires control over another, combining entities into one.
Acquisition Cost
The total cost associated with obtaining an asset, including the purchase price and any additional expenses necessary to bring it to its intended use.
Q5: How much work is done on the
Q11: A flask contains 1.00 mole of oxygen
Q15: On a cold day (-3 °C), the
Q15: Determine the electric potential at the point
Q21: If a +2.0 × 10<sup>-</sup><sup>5</sup> C point
Q32: Which one of the following phrases best
Q36: What is the rms value of the
Q40: Determine the magnitude of the electric field
Q40: A long, straight wire carries a 6.0-A
Q44: Complete the following statement: Momentum will be