An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.
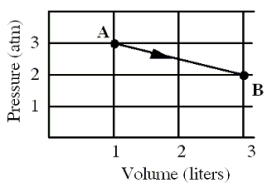
-Suppose that the same gas is originally in state A as described above,but its volume is increased isothermally until a new volume of 3.0 liters is reached.Which one of the following statements for this isothermal process is false?
Definitions:
Corporate Opportunity
A business opportunity or prospect that a company has the right to pursue, typically relevant in conflicts of interest situations.
Doctrine
A set of beliefs, policy, or position, often held by organizations, governments, or religious groups.
Prospectus
An official document issued by companies that are offering securities for sale to the public, detailing the investment’s objectives, risks, and financial statements.
Securities
Financial instruments that represent an ownership position, a creditor relationship, or rights to ownership, such as stocks, bonds, and options.
Q1: Which one of the following statements is
Q8: Two motorcycles are traveling in opposite directions
Q8: Two pulses of identical shape travel toward
Q23: A positive point charge is placed at
Q24: What is the temperature at the interface
Q27: A fan rotating with an initial angular
Q39: What is the direction of the electric
Q54: Which one of the following statements best
Q55: What is the peak voltage?<br>A)10 V<br>B)60 V<br>C)120
Q82: What is the equivalent resistance between