In a reversible heat engine, one mole of an ideal gas is carried through a circular cycle beginning and ending at point A as shown in the figure. Which one of the following statements concerning this system is false? 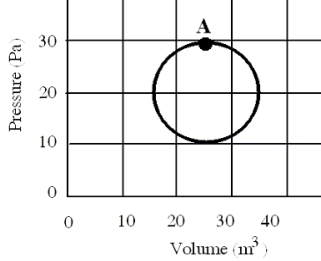
Definitions:
Simple Analysis
The process of examining data or statistical results using straightforward, basic methods without complex or advanced techniques.
One or More
An expression indicating at least one or possibly more of something.
T
Often refers to a t-score, used in various statistical analyses to compare two means and determine if they significantly differ from each other.
Cohen's d
A measure of the size of an effect, expressed as the difference between two means divided by the standard deviation, used in power analysis and meta-analysis.
Q10: Determine the magnitude of the magnetic field
Q10: The density of mercury is 1.36 ×
Q21: After a moving van drives onto a
Q31: An ideal gas is contained in a
Q31: A 0.050-kg lump of clay moving horizontally
Q35: Two uncharged, conducting spheres, A and B,
Q38: In a certain clock, a pendulum of
Q40: Which one of the following statements is
Q55: One end of a rope is tied
Q98: A 3.5-A current is maintained in