Consider the following van der Waals coefficients: 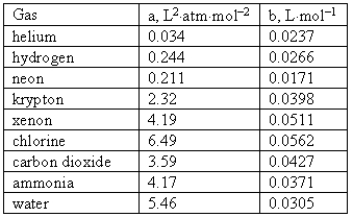 Which of the following gases has the largest attractive forces?
Which of the following gases has the largest attractive forces?
Definitions:
Ownership Disputes
Conflicts or disagreements over who holds the legal rights or title to a piece of property, asset, or intellectual property.
Limited Liability Company
A business structure that combines the pass-through taxation of a partnership or sole proprietorship with the limited liability of a corporation.
Operating Agreement
A document that outlines the governance and business operations of an LLC, agreed upon by its members.
Limited Liability Company
A business structure that combines the pass-through taxation of a partnership with the limited liability protections of a corporation.
Q1: What does the term "black body" signify
Q12: If an isolated system contained +5 kJ
Q25: How is steel defined?<br>A)A heterogeneous iron alloy
Q30: A photon with a frequency of 1.2
Q31: Which of the following has the largest
Q37: Light of wavelength 242 nm ionizes a
Q56: The condition called the bends results from
Q66: What is the density of xenon gas
Q83: Which is a feature of solid electrolytes?<br>A)Electrons
Q93: The sublimation of solid carbon dioxide is