Consider the following van der Waals coefficients: 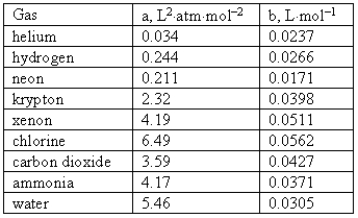 Which of the following gases has the smallest attractive forces?
Which of the following gases has the smallest attractive forces?
Definitions:
Good Faith
Acting with honesty and sincerity without any intention to deceive or defraud in a contractual or legal context.
Destructive Bargaining
Negotiation tactics that seek to undermine or diminish the position of the other party, rather than working toward mutual benefit.
Contract Negotiations
The process of discussing terms and details between parties in order to reach a mutual agreement for a contract.
First Amendment
Part of the Bill of Rights in the United States Constitution, protecting freedoms of speech, religion, press, assembly, and petition.
Q2: Which of the following species is isoelectronic
Q12: Public criticism of psychological research seems to
Q13: Which of the following is likely to
Q29: Which of the following has bond angles
Q48: A student conducts a search of the
Q52: The NCO bond angle in formamide,H<sub>2</sub>NCHO,is _.
Q53: When a mole of photons possesses an
Q68: Water was found to rise to a
Q69: How many lone pairs of electrons are
Q87: What is the vapor pressure of geraniol,molar