Calculate the standard reaction enthalpy for the following reaction: 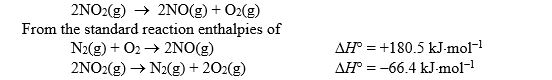
Definitions:
Cell
The basic structural, functional, and biological unit of all known living organisms.
Proteins
Large, complex molecules essential for the structure, function, and regulation of the body's cells, tissues, and organs.
23rd Pair
Refers to the pair of chromosomes that determine the biological sex of an individual in humans, commonly known as the sex chromosomes (XX for females and XY for males).
Base Pairs
The pairs of nitrogenous bases (adenine with thymine, and guanine with cytosine) that connect the two strands of a DNA molecule.
Q5: How many moles of KOH(s)must be added
Q10: How is bonding in solids best described?<br>A)Bands
Q29: True or false: the pH of 0.10
Q32: Which of the following statements is true?<br>A)Labile
Q36: Glycerol,C<sub>3</sub>H<sub>8</sub>O<sub>3</sub>,has a higher viscosity than propanol,C<sub>3</sub>H<sub>8</sub>O.True or
Q54: Which of the following would have the
Q58: Consider the reaction 3Fe(s)+ 4H<sub>2</sub>O(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1039/.jpg"
Q60: Calculate the solubility product of calcium hydroxide
Q64: Which of the following are heterogeneous alloys?<br>A)Mercury
Q73: Bond polarity tends to dominate the trend