What is the highest temperature that the substance shown in the following phase diagram can exist as a liquid? 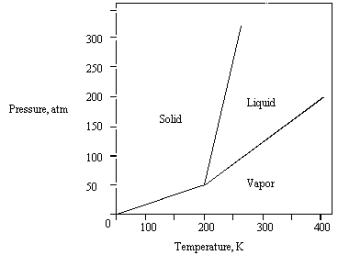
Definitions:
Q3: Consider the following reaction: 4KO<sub>2</sub>(s)+ 2CO<sub>2</sub>(g)<font face="symbol"></font>
Q9: For the reaction cyclobutane(g)<font face="symbol"></font> 2ethylene(g)at 800
Q10: Calculate the equilibrium constant for the reaction
Q20: What is the pH at the stoichiometric
Q22: The reaction 2NO(g)+ 2H<sub>2</sub>(g)<font face="symbol"></font> N<sub>2</sub>(g)+ 2H<sub>2</sub>O(g)<br>Is
Q25: Consider the following cell:<br>Zn(s)<font face="symbol"></font>Zn<sup>2+</sup>(aq,0.200 M)m H<sup>+</sup>(aq,?)<font
Q41: The experimental value of the molar entropy
Q56: Consider the compounds PCl<sub>5</sub>(g),HCN(g),CuO(s),NO(g),NH<sub>3</sub>(g),and SO<sub>2</sub>(g).<br>Which compound will
Q88: Water and acetone,CH<sub>3</sub>COCH<sub>3</sub>,both freeze at a higher
Q95: In solution based methods of nano-material fabrication,what