Given: C(s) + CO2(g) 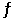 2CO(g) At equilibrium at a certain temperature,the partial pressures of CO(g) and CO2(g) are 1.22 atm and 0.780 atm,respectively.Calculate the value of K for this reaction.
2CO(g) At equilibrium at a certain temperature,the partial pressures of CO(g) and CO2(g) are 1.22 atm and 0.780 atm,respectively.Calculate the value of K for this reaction.
Definitions:
Equilibrium Price
The charge where the amount of merchandise supplied equals the amount consumers are willing to purchase.
Inelastic Demand
A situation where the demand for a product does not significantly change with a change in price.
Elastic Supply
A situation where the supply of a good changes significantly when its price changes.
Tax Burden
The measure of the impact of taxation on an individual's or entity's income, assets, or purchasing power.
Q2: Which has the higher boiling point,HCl or
Q15: How does an electron well form in
Q27: A plot of ln(vapor pressure)versus 1/T for
Q30: For the equilibrium CaCO<sub>3</sub>(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1039/.jpg" alt="For
Q56: Which of the following ions forms only
Q72: The reaction 3C + D <font face="symbol"></font>
Q73: What does "sintering" mean?<br>A)Mixing finely divided metal
Q77: The lattice enthalpy of calcium oxide is
Q84: For the cell diagram Cd(s)<font face="symbol"></font>CdSO<sub>4</sub>(aq)<font face="symbol"></font>Hg<sub>2</sub>SO<sub>4</sub><font
Q164: What property of lithium makes the lithium-ion