Given: C(s) + CO2(g) 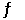 2CO(g) At equilibrium at a certain temperature,the partial pressures of CO(g) and CO2(g) are 1.44 atm and 0.820 atm,respectively.Calculate the value of K for this reaction.
2CO(g) At equilibrium at a certain temperature,the partial pressures of CO(g) and CO2(g) are 1.44 atm and 0.820 atm,respectively.Calculate the value of K for this reaction.
Definitions:
Miller and Scholes
Refers to economists Merton Miller and Myron Scholes, noted for their contributions to the field of finance, including work on the pricing of options and corporate finance.
Nonsystematic Risk
The portion of investment risk that is unique to an individual asset or company, such as management decisions or product recalls, and can be mitigated through diversification.
Security Market Line
A graphical representation of the expected return of investments at different levels of risk, showing the relationship between risk and return in the capital asset pricing model.
Security Market Line
A graphical representation used in the Capital Asset Pricing Model (CAPM) that shows the relationship between the expected return of an investment and its risk.
Q13: The equation that represents K<sub>a2</sub> for phosphoric
Q23: Given the half reaction: NO<sub>3</sub><font face="symbol"><sup></sup></font>(aq)<font face="symbol"></font>
Q26: For the reaction 2C(s)+ 2H<sub>2</sub>(g)<font face="symbol"></font> C<sub>2</sub>H<sub>4</sub>(g)<br><font
Q27: Diborane has<br>A)2 bridging hydrogens and 4 terminal
Q51: For HF,pK<sub>a</sub> = 3.45.What is the pH
Q54: Using a mass of 500.0 g of
Q68: The products of the electrolysis of CuSO<sub>4</sub>(aq)are<br>A)H<sub>2</sub>(g)and
Q68: The following 0.1 M aqueous solutions are
Q78: At the normal boiling point of chlorine,238.5
Q81: A buffer solution contains 0.25 M NaNO<sub>2</sub>(aq)and