Given: 4NH3(g) + 5O2(g) 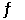 4NO(g) + 6H2O(g)
4NO(g) + 6H2O(g)
K
Calculate the equilibrium constant for the following reaction.
2NO(g) + 3H2O(g) 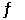 2NH3(g) +
2NH3(g) + 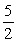 O2(g)
O2(g)
Definitions:
Budget At Completion
The total budgeted cost of a project at its completion, including all planned expenditures and liabilities.
Request For Proposal
A formal document issued by an organization inviting suppliers to submit proposals for supplying goods or services.
SPI Value
The Schedule Performance Index, a measure used in project management to compare the work performed against the work scheduled.
Earned Value Management
A project management technique that combines measurements of project scope, schedule, and cost performance to assess project progress and performance.
Q5: Molecular solids are held together by weak
Q27: For a solution of phosphoric acid,write the
Q27: The equilibrium constant,K<sub>c</sub>,for the reaction 2NOCl(g) <img
Q30: If the pK<sub>a</sub> of acetic acid is
Q33: Calculate <font face="symbol"></font>G at 298 K for
Q38: In the cell shown above,A is a
Q42: What mass of ethanol,C<sub>2</sub>H<sub>5</sub>OH(l),must be burned to
Q55: If a 1-L flask containing D<sub>2</sub>(g),N<sub>2</sub>(g),and ND<sub>3</sub>(g)at
Q63: What mass of propane,C<sub>3</sub>H<sub>8</sub>(g),must be burned to
Q94: If the pK<sub>a1</sub> = 1.81,and pK<sub>a2</sub> =