A mixture consisting of 0.250 M N2(g) and 0.500 M H2(g) reaches equilibrium according to the equation below: N2(g) 3H2(g) 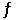 2NH3(g)
2NH3(g)
At equilibrium,the concentration of ammonia is 0.150 M.Calculate the concentration of N2(g) at equilibrium.
Definitions:
Crystalline Polymers
Polymers characterized by a highly ordered structure resulting in sharp melting points and often increased strength and rigidity.
Plasticizer
A substance added to a material to increase its flexibility or fluidity.
Crystallinity
A measure of the degree to which a material has a well-ordered, repeating atomic structure.
Ordered Rankings
A method of arranging data points or items based on their value or importance in an ascending or descending sequence.
Q2: The titration curve for the titration of
Q3: Draw a graph of the molar Gibbs
Q20: The reaction profile for the mechanism NO<sub>2</sub>(g)+
Q25: Consider the following cell:<br>Zn(s)<font face="symbol"></font>Zn<sup>2+</sup>(aq,0.200 M)m H<sup>+</sup>(aq,?)<font
Q32: Cooper pairs are formed in what way?<br>A)Electrons
Q35: The rates of first-order and second-order reactions
Q50: Write the autoprotolysis reaction for liquid ammonia.
Q59: When barium hydroxide is dissolved in water,the
Q91: Calculate <font face="symbol"></font>G<sub>r</sub><font face="symbol"></font> for the decomposition
Q91: The osmotic pressure of 1.00 g of