A mixture consisting of 0.250 M N2(g) and 0.500 M H2(g) reaches equilibrium according to the equation below: N2(g) 3H2(g) 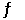 2NH3(g)
2NH3(g)
At equilibrium,the concentration of ammonia is 0.150 M.Calculate the concentration of H2(g) at equilibrium.
Definitions:
Formal Authority
Describes the power granted to an individual or position through a formal structure or hierarchy within an organization to make decisions and command obedience.
Conflict Resolution
The process of resolving disputes or disagreements between parties through dialogue, negotiation, or other methods to reach an agreement.
Forcing
A conflict resolution method where one party insists on their position without regard to the impact on the other parties involved.
Problem Solving
The process of identifying a challenge and developing strategies to address it effectively.
Q5: How many moles of KOH(s)must be added
Q8: The following 0.1 M aqueous solutions are
Q13: What is the half-life of a reaction
Q16: When the Ag(s)<font face="symbol"></font>AgCl(s)<font face="symbol"></font>Cl<font face="symbol"><sup></sup></font>(aq)electrode acts
Q17: The standard potential of the cell Ag(s)<font
Q52: In a system composed of nitrogen gas
Q53: How much work is done by a
Q61: The combustion of 1 mole of ethanol,C<sub>2</sub>H<sub>5</sub>OH(l),in
Q79: Ferrofluids contain suspensions of coarsely divided elemental
Q89: Which of the following compounds is predicted