Consider the reaction below:
F2(g) 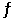 2F(g)
2F(g)
(a)Compressing the reaction mixture results in a change in Q.True or false?
(b)Heating the reaction mixture causes the reaction to shift to the left.True or false?
(c)At 1000 K,the equilibrium constant for the reaction is about 104.If the reaction is perturbed such that Q = 1,the reaction must shift to the left.True or false?
Definitions:
Wages And Salaries
Remuneration provided to workers for their work, encompassing both wages by the hour and set salaries.
Spending Variance
A financial metric indicating the difference between the budgeted or planned amount of expenses and the actual amount spent.
Net Operating Income
A company's profit after subtracting its operating expenses, excluding taxes and interest.
Revenue And Spending Variances
The differences between actual and budgeted amounts for revenue and expenditures, which are analyzed to manage financial performance.
Q5: From what are ortho-silicates built?<br>A)SiO<sub>2</sub><sup>2-</sup><br>B)SiO<sub>2</sub><sup>4</sup><sup>-</sup><br>C)SiO<sub>4</sub><sup>2-</sup><br>D)SiO<sub>4</sub><br>E)SiO<sub>4</sub><sup>4</sup><sup>-</sup>
Q12: What is the end result of the
Q15: Given: PH<sub>3</sub>(g)<font face="symbol"></font> P<sub>4</sub>(s),basic solution. How many
Q16: When the Ag(s)<font face="symbol"></font>AgCl(s)<font face="symbol"></font>Cl<font face="symbol"><sup></sup></font>(aq)electrode acts
Q33: Calculate the final temperature when 2.50 kJ
Q71: The energy levels of two particle in
Q71: True or false: for the reaction<br>N<sub>2</sub>O<sub>5</sub>(s)<font face="symbol"></font>
Q85: The molar entropy of Pb(s)at 298 K
Q89: Calculate the standard reaction enthalpy for the
Q93: The following compounds are available as 0.10