Consider the following reaction at a certain temperature: 2HI(g) 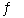 H2(g) + I2(g) K = 0.420
H2(g) + I2(g) K = 0.420
If 2.0 mol of H2(g) and I2(g) ,and 0.10 mol HI(g) were mixed in a 1.0-L flask,then
Definitions:
Irrational Appraisal
An evaluation or judgment of a situation or event that significantly distorts reality, often leading to inappropriate emotional or behavioral responses.
Albert Ellis
An American psychologist who developed Rational Emotive Behavior Therapy (REBT), a form of psychotherapy that emphasizes changing irrational beliefs.
Graduate School
An advanced program of study focused on a particular academic discipline or profession, typically following the completion of a bachelor's degree.
Rational Emotive Therapy
A form of cognitive-behavioral therapy focusing on changing irrational beliefs to alter emotional and behavioral responses.
Q26: Sodium borohydride is an oxidizing agent.True or
Q32: What is the equilibrium constant for the
Q38: The K<sub>a</sub> of phenol is 1.3 <font
Q44: Saline carbides contain the anion(s)<br>A)C<sub>2</sub><sup>2</sup><font face="symbol"><sup></sup></font> and
Q48: A possible mechanism for the reaction A
Q66: What is the [H<sup>+</sup>] for a solution
Q67: Consider the phase diagrams for water and
Q78: In a working electrochemical cell (+ cell
Q90: Give <font face="symbol"></font>H<sub>r</sub> values for each of
Q109: The equation for the hydrometallurgical extraction of