Calculate the equilibrium constant for the following reaction at 25C 2TiCl3(s) + 2HCl(g) 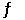 2TiCl4(g) + H2(g)
2TiCl4(g) + H2(g)
Given G = +46.6 kJ.
Definitions:
Benchmark
A standard or point of reference against which the performance of an investment, individual, or strategy can be measured.
EAFE
An acronym for Europe, Australasia, and Far East, indicating a stock index that represents major overseas equity markets.
EAFE Index
An index that represents the stock performance of developed markets outside of North America, providing a benchmark for international equity investments.
Non-U.S. Stocks
Shares of companies incorporated outside the United States available for investment.
Q18: What is the molality of carbon tetrachloride
Q21: Which of the following statements is true
Q30: Consider the following compounds and their standard
Q40: The reaction 2ClO<sub>2</sub>(g)+ F<sub>2</sub>(g)<font face="symbol"></font> 2FClO<sub>2</sub>(g)<br>Is first
Q44: A cubic close-packed structure has<br>A)A coordination number
Q61: If the rate of reaction increases by
Q68: What are the two major elements in
Q73: A system had 150 kJ of work
Q77: If the value of K<sub>b</sub> for pyridine
Q167: The formula of boric acid written as