Given: 2SO2(g) + O2(g) 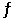 2SO3(g) At equilibrium at a certain temperature,the concentrations of SO3(g) ,SO2(g) ,and O2(g) are 0.12 M,0.86 M,and 0.33 M,respectively.Calculate the value of Kc for this reaction.
2SO3(g) At equilibrium at a certain temperature,the concentrations of SO3(g) ,SO2(g) ,and O2(g) are 0.12 M,0.86 M,and 0.33 M,respectively.Calculate the value of Kc for this reaction.
Definitions:
Parallel Form Reliability
The consistency of test scores across different versions of a test designed to be equivalent in terms of content and difficulty level.
Parallel Form Reliability
Refers to the extent to which two versions of a test yield similar results, assessing the same skills or constructs under similar conditions.
Content Validity
The extent to which a test measures all aspects of the concept or constructs it intends to measure, ensuring the test is representative of all facets of the subject.
Construct Validity
The degree to which a test or instrument accurately measures the theoretical construct or variable it is intended to measure.
Q6: The equilibrium constant expression for the reaction<br>CuSO<sub>4</sub>(s)
Q13: A buffer contains equal concentrations of NH<sub>3</sub>(aq)and
Q23: The rate law for the following mechanism
Q26: Assuming no volume change on mixing,what mass
Q43: What is the coordination number of silver
Q47: Combustion reactions can be exothermic or endothermic.True
Q49: A metal with a cubic close-packed structure
Q52: The freezing point of seawater is about
Q67: The amino acid alanine,HOOC-CH(CH<sub>3</sub>)NH<sub>3</sub><sup>+</sup>,has K<sub>a1</sub> = 4.5
Q94: What is a quantum dot?<br>A)Three-dimensional structures of