Given: C(s) + CO2(g) 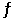 2CO(g) At equilibrium at a certain temperature,the partial pressures of CO(g) and CO2(g) are 1.22 atm and 0.780 atm,respectively.Calculate the value of K for this reaction.
2CO(g) At equilibrium at a certain temperature,the partial pressures of CO(g) and CO2(g) are 1.22 atm and 0.780 atm,respectively.Calculate the value of K for this reaction.
Definitions:
Cost Volume Profit Analysis
A financial modeling tool used to determine the effects of changes in costs and volume on a company's profits.
Sales Mix
The combination of products or services that a company sells, impacting its overall profitability.
External Factors
Elements outside a company that can impact its performance or operations, such as economic conditions, competition, and regulatory environment.
Sales Revenue
The income received by a company from its sales of goods or the provision of services before any costs or expenses are deducted.
Q32: Which of the following can form intermolecular
Q33: For a given first-order reaction,after 230 s,33%
Q42: What mass of ethanol,C<sub>2</sub>H<sub>5</sub>OH(l),must be burned to
Q55: The following compounds are available as 0.10
Q69: The pH of 0.010 M H<sub>3</sub>PO<sub>4</sub>(aq)is 2.24.Estimate
Q78: Choose the effective pH range of an
Q83: For a second-order reaction,a straight line is
Q85: What current is required to produce 91.6
Q88: Which of the following has the smallest
Q92: In a pressure cooker,the boiling point of