Given: C(s) + CO2(g) 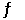 2CO(g) At equilibrium at a certain temperature,the partial pressures of CO(g) and CO2(g) are 1.44 atm and 0.820 atm,respectively.Calculate the value of K for this reaction.
2CO(g) At equilibrium at a certain temperature,the partial pressures of CO(g) and CO2(g) are 1.44 atm and 0.820 atm,respectively.Calculate the value of K for this reaction.
Definitions:
Simple Average
A simple average is a statistical measure that calculates the mean of a set of numbers by adding them together and then dividing by the count of those numbers.
Goals Against Average
A statistical measure used in sports, particularly hockey, to quantify the average number of goals a goalie allows per game.
Goalie
A player in sports, particularly in hockey or soccer, responsible for protecting the goal from being scored by the opposing team.
Shutout
In finance, it typically means being prevented from participating in an opportunity, often due to timing or fulfillment conditions not being met.
Q6: The solubility of silver chloride is greater
Q12: Consider the reaction 2N<sub>2</sub>O(g)<font face="symbol"></font> 2N<sub>2</sub>(g)+ O<sub>2</sub>(g)<br>Rate
Q24: For the reaction 2CaSO<sub>4</sub>(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1039/.jpg" alt="For
Q40: The phase diagram for a pure substance
Q66: Because a solute increases the entropy of
Q66: Calculate the average H-S bond enthalpy in
Q67: A nonsteroidal anti-inflammatory drug is metabolized with
Q74: How do the saline hydrides react in
Q82: Benzene would likely dissolve which of the
Q86: What is the pH of an aqueous