The equilibrium constant,K,for the reaction 2HgO(s) 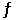 2Hg(l) + O2(g)
2Hg(l) + O2(g)
Is 1.2 1030.Calculate K for the reaction
1/2O2(g) + Hg(l) 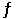 HgO(s) .
HgO(s) .
Definitions:
Petty Cash
A small amount of cash on hand used for covering minor expenses.
Balance Sheet
A financial statement that summarizes a company's assets, liabilities, and stockholders' equity at a specific point in time, providing a snapshot of its financial condition.
Cash Equivalents
Short-term, highly liquid investments that are readily convertible to known amounts of cash and which are subject to an insignificant risk of changes in value.
Short-Term Investments
Assets that are expected to be converted into cash or sold within one year or the operating cycle, whichever is longer.
Q12: Consider the reaction 2N<sub>2</sub>O(g)<font face="symbol"></font> 2N<sub>2</sub>(g)+ O<sub>2</sub>(g)<br>Rate
Q34: If the standard reaction enthalpy for the
Q39: What makes calcium carbonate such a good
Q41: At 25<font face="symbol"></font>C,K<sub>c</sub> = 1.58 <font face="symbol"></font>
Q52: In a system composed of nitrogen gas
Q57: The standard voltage of the cell Ag(s)<font
Q69: How does a p-n junction allow electric
Q80: Which of the following is not a
Q86: Which pair of metals will dissolve in
Q93: Which of the following reactions is not