Given: SO2(g) 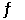 O2(g) + S(s)
O2(g) + S(s)
Kc = 2.5 1053
SO3(g) 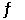
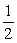 O2(g) + SO2(g)
O2(g) + SO2(g)
Kc = 4.0 1013
Calculate Kc for the reaction
2S(s) + 3O2(g) 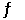 2SO3(g)
2SO3(g)
Definitions:
Employed Category
A classification within the labor force that includes all individuals who are gainfully employed, either in full-time or part-time positions.
Employed Category
A classification used in labor statistics that includes individuals who are currently working for pay or profit, or as unpaid workers in a family business.
Part-time Workers
Individuals employed in positions that require fewer hours than the standard for full-time employment, typically less than 35-40 hours a week.
Full-time Workers
Individuals who are employed in jobs that typically require a minimum number of hours worked per week, often considered to be 35-40 hours.
Q1: For gaseous hydrogen atoms at 298 K
Q17: The standard reaction free energy for the
Q27: Diborane has<br>A)2 bridging hydrogens and 4 terminal
Q33: Which of the following reactions has the
Q34: If the standard reaction enthalpy for the
Q47: What makes phosphors glow?<br>A)Electron impacts.<br>B)Plasma discharges.<sup> </sup><br>C)Irradiation
Q47: Calculate <font face="symbol"></font>S<sub>surr</sub>° at 298 K for
Q75: The addition of 125 mg of caffeine
Q86: Why is gravel often used as a
Q164: What property of lithium makes the lithium-ion