At 600C,the equilibrium constant for the reaction 2HgO(s) 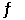 2Hg(l) + O2(g)
2Hg(l) + O2(g)
Is 2.8.Calculate the equilibrium constant for the reaction 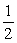 1/2O2(g) + Hg(l)
1/2O2(g) + Hg(l) 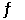 HgO(s)
HgO(s)
Definitions:
Offshoring
The relocation of business processes or manufacturing to a foreign country, typically to leverage lower costs or other benefits.
Infant Industry
A new or emerging industry that may be protected by the government from international competition to allow it to grow and establish itself.
Foreign Competition
The competitive pressure that domestic companies face from the products and services offered by foreign firms in the same market.
General Agreement on Tariffs and Trade
An international trade treaty aimed at reducing customs tariffs and other trade barriers to foster global economic trade.
Q8: What improves the corrosion resistance of brass?<br>A)Antimony.<br>B)Arsenic.<br>C)Tin.<br>D)None
Q16: Calculate the enthalpy change that occurs when
Q21: In the rock-salt structure (NaCl),the cations occupy
Q31: The following phase diagram is for a
Q33: The standard voltage of the cell Pt<font
Q50: For the reaction 2SO<sub>3</sub>(g)<font face="symbol"></font> 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)<br><font
Q60: What is the highest temperature that the
Q71: Consider the titration of 50.0 mL of
Q101: Order the following ions according to their
Q115: The anhydrous material with the common name