Given: 4NH3(g) + 5O2(g) 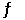 4NO(g) + 6H2O(g)
4NO(g) + 6H2O(g)
K
Calculate the equilibrium constant for the following reaction.
2NH3(g) + 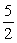 O2(g)
O2(g) 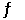 2NO(g) + 3H2O(g)
2NO(g) + 3H2O(g)
Definitions:
Materials Price Variance
The difference between the actual cost of materials used in production and the expected (or standard) cost, indicating how much more or less was spent on materials than anticipated.
Total Expenses
The sum of all costs and expenses associated with operating a business, including both fixed and variable costs.
Spending Variance
The difference between the actual amount spent and the budgeted amount for a particular cost or expense category.
Equipment Depreciation
The systematic allocation of the cost of physical assets used in production over their useful lives.
Q5: How many moles of KOH(s)must be added
Q26: For the reaction 2C(s)+ 2H<sub>2</sub>(g)<font face="symbol"></font> C<sub>2</sub>H<sub>4</sub>(g)<br><font
Q38: Of the following,which would likely dissolve in
Q46: The HBr synthesis is thought to involve
Q65: Ceramics are not brittle.True or false?
Q73: What does "sintering" mean?<br>A)Mixing finely divided metal
Q86: The reaction N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)<font face="symbol"></font> 2NH<sub>3</sub>(g)is exothermic.This
Q87: The amino acid alanine,HOOC-CH(CH<sub>3</sub>)NH<sub>3</sub><sup>+</sup>,has K<sub>a1</sub> = 4.5
Q89: Brass is an example of what kind
Q101: Order the following ions according to their