Given: P4(s) + 6Cl2(g) b 4PCl3(l)
K
Calculate the equilibrium constant for the following reaction.
2PCl3(l) 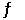 3Cl2(g) +
3Cl2(g) + 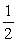 P4(s)
P4(s)
Definitions:
Vocational Rehabilitation Act
A federal law enacted in 1973 designed to help individuals with disabilities access rehabilitation services to gain employment, enhancing their economic independence.
Employment Discrimination
Unfair treatment of employees or job applicants based on race, gender, age, religion, national origin, disability, or other protected characteristics.
Alcoholics
Individuals who suffer from alcoholism, a chronic disease characterized by uncontrolled drinking and a preoccupation with alcohol.
Commitment Systems
Frameworks or strategies employed by organizations to enhance employee engagement and dedication towards the company's goals and values.
Q5: From what are ortho-silicates built?<br>A)SiO<sub>2</sub><sup>2-</sup><br>B)SiO<sub>2</sub><sup>4</sup><sup>-</sup><br>C)SiO<sub>4</sub><sup>2-</sup><br>D)SiO<sub>4</sub><br>E)SiO<sub>4</sub><sup>4</sup><sup>-</sup>
Q9: If the standard potential for Cu<sup>2+</sup>(aq)/Cu<sup>+</sup>(aq)is 0.15
Q12: Consider the reaction 2N<sub>2</sub>O(g)<font face="symbol"></font> 2N<sub>2</sub>(g)+ O<sub>2</sub>(g)<br>Rate
Q16: The equation that corresponds to the K<sub>a</sub>
Q22: Consider the reaction 2Fe<sub>2</sub>O<sub>3</sub>(s)+ 3C(s)<font face="symbol"></font> 4Fe(s)+
Q31: For the cell diagram<br>Pt<font face="symbol"></font>H<sub>2</sub>(g)<font face="symbol"></font>H<sup>+</sup>(aq)mCo<sup>3+</sup>(aq),Co<sup>2+</sup>(aq)<font face="symbol"></font>Pt<br>write
Q38: The reaction 2NO<sub>2</sub>(g)<font face="symbol"></font> 2NO(g)+ O<sub>2</sub>(g)<br>Is postulated
Q38: In the cell shown above,A is a
Q52: The freezing point of seawater is about
Q79: The pH of 0.010 M H<sub>3</sub>PO<sub>4</sub>(aq)is 2.24;