A mixture consisting of 0.250 M N2(g) and 0.500 M H2(g) reaches equilibrium according to the equation below: N2(g) 3H2(g) 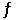 2NH3(g)
2NH3(g)
At equilibrium,the concentration of ammonia is 0.150 M.Calculate the concentration of N2(g) at equilibrium.
Definitions:
Epinephrine
A hormone produced by the adrenal medulla.
Mottled Blueness
A condition characterized by patchy blue discoloration of the skin, often due to poor oxygenation or circulation.
Partial-thickness
A classification of wounds or burns that extend through the epidermis and possibly into, but not through, the dermis, indicating a certain depth but not complete thickness involvement.
BSA
An abbreviation often used for Body Surface Area, a measure of the total area of the human body used for dosing of certain medications.
Q1: Which of the following occurs when HNO<sub>3</sub>(aq),Cu(s),and
Q4: Which answer best accounts for the Cl<font
Q10: At 25<font face="symbol"></font>C,<font face="symbol"></font>G<sub>r</sub><sup>o</sup> for the reaction
Q16: How is conduction in metals and graphite
Q30: A first-order reaction has a rate constant
Q36: In the thermite reaction,iron (III)oxide is the
Q44: The rate law for the following mechanism
Q51: For HF,pK<sub>a</sub> = 3.45.What is the pH
Q77: The reaction [(CN)<sub>5</sub>CoOH<sub>2</sub>]<sup>2</sup><font face="symbol"><sup></sup></font>(aq)+ SCN<font face="symbol"><sup></sup></font>(aq)<font face="symbol"></font>
Q92: Which of the following would have the