Consider the reaction PCl5(g) 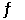 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
At a certain temperature,if the initial concentration of PCl5(g) is 2.0 M,at equilibrium the concentration of Cl2(g) is 0.30 M.Calculate the value of Kc at this temperature.
Definitions:
Corporate Bonds
Debt securities issued by corporations to finance their operations, which typically pay periodic interest payments and return the principal at maturity.
Common Stock
A security that evidences ownership in a corporation. A share of common stock gives the owner a proportionate interest in the corporation with regard to control, earnings, and net assets. Common stock is lowest in priority with respect to payment of dividends and distribution of the corporation’s assets on dissolution.
Proportionate Interest
The share or percentage ownership that an individual or entity holds in a particular asset, reflecting their portion of the total value or earnings.
Corporate Veil
A legal concept that separates the actions of a corporation from its shareholders, protecting them from being personally liable for the company's debts and obligations.
Q4: If the molar solubility of the compound
Q5: What is the formula of baking soda?<br>A)Na<sub>2</sub>CO<sub>3</sub><font
Q9: If the K<sub>sp</sub> of AgI is 1.5
Q13: At constant pressure and temperature,which of the
Q19: Calculate the number of moles of oxygen
Q20: Match the values with the correct enthalpy
Q25: The increase in entropy of the system
Q47: What is the value of E for
Q69: The density of sodium metal is 0.97
Q86: What is the total motional contribution to