Consider the reaction PCl5(g) 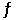 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
At a certain temperature,if the initial concentration of PCl5(g) is 3.0 M,at equilibrium the concentration of Cl2(g) is 0.80 M.Calculate the value of Kc at this temperature.
Definitions:
Ethics Of Training
Guidelines and principles that govern the fairness, respect, and integrity of training processes and practices.
Ethical Guidelines
A set of principles designed to guide decision-making and conduct when addressing moral issues in a professional context.
Development
The process of economic, social, and technological progression and improvement in a society or an organization.
Training Delivery Problems
Challenges or obstacles that impede the effective conveyance of training content.
Q3: What equation corresponds to the standard enthalpy
Q4: A certain reaction has a rate constant
Q12: What is the end result of the
Q12: The temperature of 2.00 mol Ne(g)is increased
Q36: Of the following solutes,which is likely to
Q38: Of the following,which would likely dissolve in
Q49: Calculate <font face="symbol"></font>G for the process<br>He(g,1 atm,298
Q57: Upon what are silicates based?<br>A)Repeating silicon chains.<br>B)Repeating
Q77: The ability of a semi-conductor to carry
Q91: For a substance to be considered a