Consider the reaction below:
F2(g) 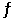 2F(g)
2F(g)
(a)Compressing the reaction mixture results in a change in Q.True or false?
(b)Heating the reaction mixture causes the reaction to shift to the left.True or false?
(c)At 1000 K,the equilibrium constant for the reaction is about 104.If the reaction is perturbed such that Q = 1,the reaction must shift to the left.True or false?
Definitions:
Enrolled
Definition: Being officially registered and participating in a course, program, or institution.
Anticipated Regret
The emotional distress one expects to feel from a potential future outcome of making a particular choice.
Decision Avoidance
A behavior or tendency to defer, avoid, or abandon making choices to avert potential negative outcomes or regret.
Certainty Effect
The tendency for people to overvalue outcomes that are certain compared to those that are only probable.
Q3: The concentration<font face="symbol"></font>time dependence for a first-order
Q5: Which of the following is true for
Q17: True or false: the van't Hoff i
Q25: The increase in entropy of the system
Q39: You have available the following reagents as
Q43: The equation that represents K<sub>a2</sub> for sulfurous
Q79: Consider the following cell: Ag(s)<font face="symbol"></font>Ag<sup>+</sup>(aq,0.100 M)mAg<sup>+</sup>(aq,0.100
Q86: The reaction N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)<font face="symbol"></font> 2NH<sub>3</sub>(g)is exothermic.This
Q91: Calculate <font face="symbol"></font>G<sub>r</sub><font face="symbol"></font> for the decomposition
Q93: How is pig iron produced?<br>A)In a charcoal