Consider the reaction 4NH3(g) + 3O2(g) 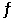 2N2(g) + 6H2O(g) ,K = 1080 at a certain temperature.
2N2(g) + 6H2O(g) ,K = 1080 at a certain temperature.
Initially,all reactants and products have concentrations equal to 12 M.At equilibrium,the approximate concentration of oxygen is
Definitions:
1880
A year that marked the early stages of the Industrial Revolution in America, significant immigration, and the continuation of the post-Civil War Reconstruction era.
Spanish-American War
A conflict in 1898 between Spain and the United States, resulting from U.S. intervention in the Cuban War of Independence, which led to the U.S. acquiring territories in the western Pacific and Latin America.
Open Door Policy
A diplomatic concept aimed at promoting equal opportunities for international trade and commerce for all nations, particularly in relation to China in the late 19th and early 20th centuries.
Q8: Calculate the standard free energy of formation
Q9: What contaminant causes the red "terra cotta"
Q9: If the standard potential for Cu<sup>2+</sup>(aq)/Cu<sup>+</sup>(aq)is 0.15
Q17: True or false: the van't Hoff i
Q20: Match the values with the correct enthalpy
Q44: What is a magnetic domain?<br>A)A magnetic field.<br>B)A
Q60: For the reaction A <font face="symbol"></font> products,the
Q61: Write the equilibrium constant for 2NaBr(aq)+ Pb(ClO<sub>4</sub>)<sub>2</sub>(aq)
Q82: The solubility of silver bromide is greater
Q116: The reaction of potassium superoxide with water